 I have got chemistry on the brain thanks to my latest writing project. There are atoms and isotopes, molecules and compounds all floating around, creating some pretty wild dreams!
I have got chemistry on the brain thanks to my latest writing project. There are atoms and isotopes, molecules and compounds all floating around, creating some pretty wild dreams!
So, I thought that you all should reap the benefits of my writing process!
In today’s post, I am sharing a bit about the history of the atom and its structure, as well as explaining isotopes. I have also added in a game and several activities to help you wrap it all up.
Let’s get started…
What are Atoms and Isotopes?
History of the Atom
The Greeks were the first to discuss the concept of an atom. They believed that matter could be cut into smaller and smaller pieces, but that eventually you would get to a piece that could not be cut. So, the word atom comes from the Greek word, atomos, which means “uncuttable”.
It wasn’t until 1808 that John Dalton, an English scientist and schoolteacher, developed a theory about the how atoms behave. His theory said that an element is composed of tiny particles called atoms, in an ordinary chemical reaction, no atom of an element disappears and compounds are formed when atoms of two or more elements combine.
So on the basis of his theory; scientists were now able to say that the atom was the smallest particle of an element. Modern atomic theory is very similar to what Dalton proposed, except now we know the sub-particles that compose an atom as well as that structure of an atom. So, let’s take a closer look at the structure of an atom.
The Structure of an Atom
The atom is composed of three smaller subatomic particles, called the proton, neutron and electron. The proton is a positively charged particle that resides in the nucleus at the center of an atom. The neutron is a particle with no charge that also resides in the nucleus of an atom. The electron is a negatively charged particle that resides in a cloud around the nucleus, which is called an electron shell.
Atoms have an equal number of protons and electrons, which gives them no net charge. In other words, the positive charges from the protons are cancelled out by the negative charges of the electrons within the atom. Generally an atom of a given element has the same number of neutrons as protons, but there are exceptions.
Isotopes
Some atoms have additional neutrons in their nucleus, and we call these atoms isotopes of the element. These isotopes have the same atomic number, but different atomic mass. Remember that the atomic number is the number of protons in an element and atomic mass is the total weight of the protons, neutrons and electrons in an element. So it makes sense that an isotope would have the same atomic number, but a different atomic mass from the original element.
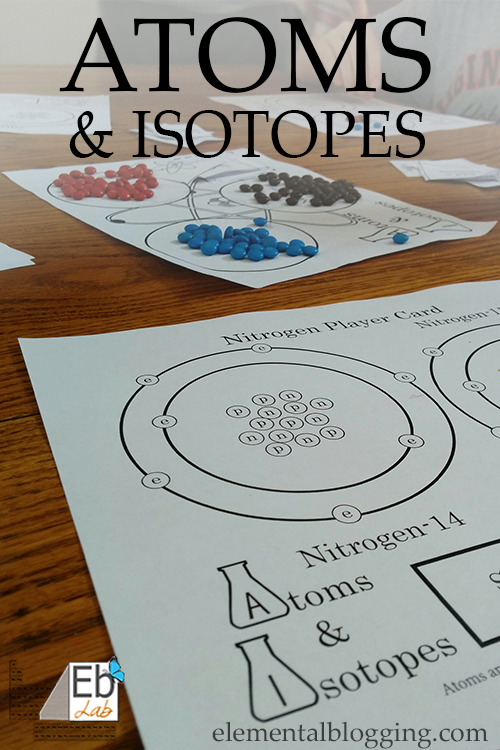
Atoms and Isotopes Game
We had a blast playing this game as a family, even our 5 year old understood how to play. It’s a great way to sneak in some super-fun review after you have learned about atoms and isotopes!
Supplies
- Blue, brown, and red colored beads or mini-M&M’s (each player will need about 15 of each color)
- Atoms & Isotopes Game Board, Cards, and Player Cards (Download for Free)
How to Play
- Set up the game.
- Sort the beads or mini-M&M’s into the three piles on the game board – electrons (blue), protons (red), and neutrons (brown).
- Place the game cards in the provided square.
- Choose to do an easy game (hydrogen card) or challenging game (nitrogen card). Hand the appropriate player card to each player.
- Read the rules of the game.
- Electrons go on the circles marked with an “e” on the player card, protons go on the circles marked with a “p”, and neutrons go on the circles marked with a “n” on the player card.
- Players can fill the space on either atom until both are complete. If they do not have space for the subatomic particle they have chosen, the particle is place in their holding tank at the bottom of the player card.
- Players may use the subatomic particles in the holding tank to fill the spaces left when they choose a card that tells them to lose a particle.
- The youngest player begins the game by choosing a card and then doing what it tells you to do. Once they are done, the player to the right chooses a card and repeats the process.
- Game play continues until someone wins.
How to Win
Be the first player to create an atom of each of the stable isotopes on their player card.
More Atoms and Isotopes Fun
As you work through this information with your students, you can have them create a narration sheet using the template below:
- Atoms and Isotopes Narration Sheet {FREE Download}
Here are a few yummy atom models you can make with your students:
Here are a few books you can check out from the library to learn more about atoms:
- What Are Atoms? (Rookie Read-About Science) by Lisa Trumbauer
- Atoms and Molecules (Building Blocks of Matter) by Richard and Louise Spilsbury
- Atoms (Simply Science) by Melissa Stewart
 Sign up below to receive weekly tips & tools for homeschool science and we'll send you a FREE copy of
Sign up below to receive weekly tips & tools for homeschool science and we'll send you a FREE copy of 